Plated busbars with function
In electroplating, direct electric current is passed through an electrolytic bath. The metal ions dissolved in the electrolytic bath are deposited on the busbar by reduction, thus sealing its busbar. This process is often used to make busbars even more conductive and to protect them from corrosion. With our experience in this field, many things are possible. Thanks to our expertise, we can always offer you the right coating for your system solution – contact us now!

The EMS Group is certified according to
DIN EN ISO 9001:2015
Plated busbars for more corrosion resistance

Galvanic silver plated busbars
At high contact temperatures, but also in chemically very aggressive atmospheres, silver is used as corrosion protection.
Silver-plated busbars have the most electrically conductive coating because the electrons of the silver plating can move more freely than those of other precious metals. Thus, silver has a high thermal conductivity.
Galvanic gold plated busbars
Gold plating increases the longevity of busbars and protects them from chemical or mechanical influences.
Gold also has much higher corrosion resistance than silver, but is less conductive than silver.


Tinned busbars
Tin is often used as a cost-effective protective layer for contact surfaces. The tin-plated surface is produced either by electroplating or by dipping.
In the case of busbars for e-mobility, for example, care should be taken to ensure permanently low contact resistances at the connection points. Tinned surfaces ensure these permanently low contact resistances. At the same time, tin plating prevents oxidation of copper.
Galvanically or chemically nickel-plated busbars
Nickel is resistant to air, water, dilute acids and most alkalis and offers very good long-term stability as a contact surface.
Nickel-plated busbars therefore have high corrosion resistance, making them particularly well suited for many harsh environment applications. In addition, nickel-plated busbars have good thermal conductivity and very good electrical conductivity.

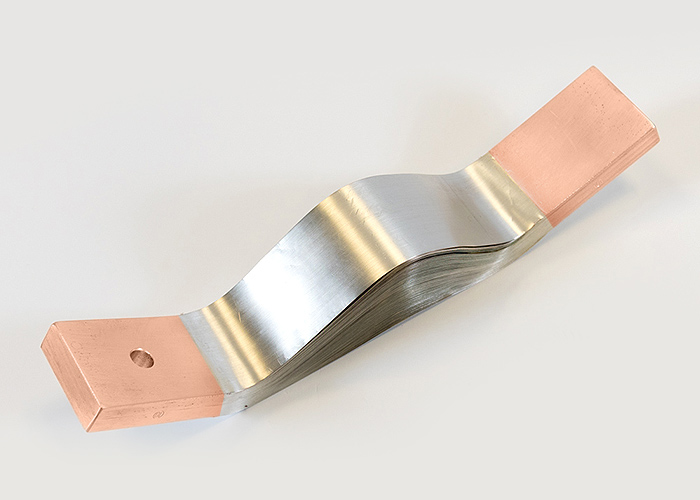
Copper plated busbars
Copper has excellent conductivity. Copper-plating the contact surfaces of aluminum components facilitates contact with other copper parts. At the transition from an aluminum rail, for example, a screw connection can be made with the same metals (Cu on Cu). Therefore, we offer a wide range of possibilities:
- Explosive cladding is a process in which copper and aluminum are bonded together by the pressure of a controlled explosion.
- Galvanic copper plating.
- Cold spraying is a plating process in which copper in powder form is applied at very high speed to the mostly aluminum components, either over the entire surface or partially.